Standard air has a specific heat value of, well, it depends! Seriously though, understanding the specific heat of standard air is crucial in fields ranging from HVAC design to aerospace engineering. We’re talking about the amount of energy needed to change the temperature of a given mass of air – a seemingly simple concept with surprisingly complex implications.
This exploration dives into the nitty-gritty of defining “standard air,” calculating its specific heat at constant pressure and volume, and examining how factors like humidity and temperature influence these values. Buckle up, it’s gonna get a little nerdy.
We’ll cover the composition of standard air, explore the difference between constant pressure (Cp) and constant volume (Cv) specific heats, and even tackle some real-world applications. Think HVAC system calculations, aerospace engineering challenges, and meteorological modeling – all of which rely heavily on accurate specific heat data. Plus, we’ll compare standard air to other gases and work through an example of an adiabatic process to really solidify your understanding.
Specific Heat Capacity of Standard Air
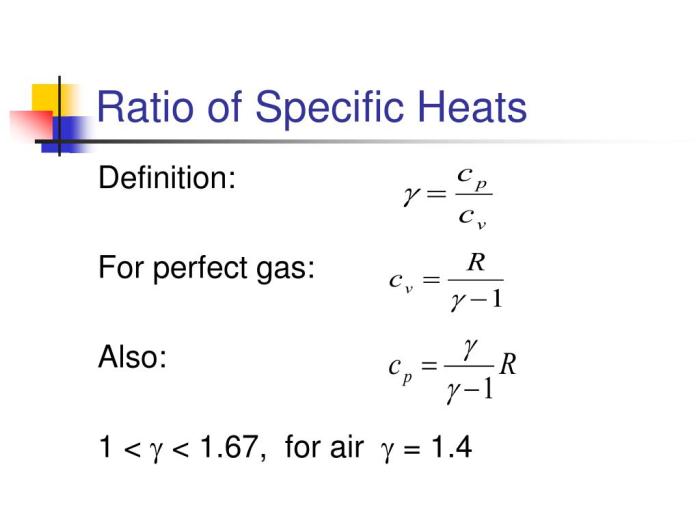
Understanding the specific heat capacity of air is crucial in various engineering applications, from designing HVAC systems to analyzing atmospheric processes. This property dictates how much energy is needed to change the temperature of a given mass of air. This discussion will delve into the nuances of specific heat capacity, focusing on the distinctions between constant pressure and constant volume scenarios.Specific Heat Capacity: Constant Pressure vs.
Constant VolumeSpecific heat capacity quantifies the amount of heat required to raise the temperature of one kilogram of a substance by one degree Celsius (or one Kelvin). However, the value of specific heat capacity depends on the conditions under which the heating process occurs. For air, we primarily distinguish between specific heat at constant pressure (Cp) and specific heat at constant volume (Cv).
When heating at constant pressure, the air can expand, performing work against its surroundings. This means more energy is required to raise the temperature compared to heating at constant volume, where no expansion work is done. Therefore, Cp is always greater than Cv.Units of Specific Heat CapacitySpecific heat capacity is typically expressed in units of Joules per kilogram-Kelvin (J/kg·K) or kilojoules per kilogram-degree Celsius (kJ/kg·°C).
These units reflect the energy required per unit mass per unit temperature change. Both units are equivalent since a change of 1 Kelvin is equal to a change of 1 degree Celsius.
Specific Heat Capacity of Standard Air at Various Temperatures, Standard air has a specific heat value of
The specific heat capacity of air is not constant; it varies slightly with temperature. The following table shows approximate values for standard air (dry air) at different temperatures. Note that these values are approximations, and more precise values may be needed for specific applications. These values are typically obtained from thermodynamic property tables or correlations based on experimental data.
| Temperature (°C) | Cp (kJ/kg·°C) | Cv (kJ/kg·°C) | Difference (Cp – Cv) (kJ/kg·°C) |
|---|---|---|---|
| 0 | 1.005 | 0.718 | 0.287 |
| 20 | 1.005 | 0.718 | 0.287 |
| 100 | 1.008 | 0.721 | 0.287 |
| 500 | 1.03 | 0.743 | 0.287 |
Note: The difference (Cp – Cv) is approximately equal to the gas constant for air (R = 0.287 kJ/kg·K). This relationship is derived from thermodynamic principles and is a useful check on the consistency of the data.
Factors Affecting Specific Heat Capacity

Okay, so we’ve established what the specific heat capacity of standard air is. But, like, it’s not alwaysthat* value. Real-world air is way more complex, and several factors can tweak that number. Let’s dive into what those are.
The specific heat capacity of air isn’t a fixed constant; it’s influenced by its composition and the surrounding conditions. Think of it like this: adding different ingredients to a recipe changes the final dish’s properties. Similarly, altering air’s makeup or its environment alters its heat-holding capacity.
Air Composition and Humidity
Humidity, or the amount of water vapor in the air, significantly impacts the specific heat capacity. Water vapor has a higher specific heat capacity than dry air. Therefore, more humid air will have a higher specific heat capacity than drier air. This is because water molecules can absorb and store more energy than the molecules in dry air (primarily nitrogen and oxygen).
For instance, a humid summer day will require more energy to raise the temperature compared to a dry desert day. The effect isn’t huge, but it’s measurable and important for accurate calculations, especially in weather forecasting and climate modeling.
Temperature’s Effect on Specific Heat Capacity
The specific heat capacity of air isn’t completely constant even at a fixed composition. It shows a slight dependence on temperature. As temperature increases, the specific heat capacity of air tends to increase slightly. This is because the molecular vibrations and rotational energies become more significant at higher temperatures, leading to increased energy absorption for a given temperature change.
The change isn’t dramatic, but it’s important for high-precision applications where even small variations matter. Think about the difference in heating a room in winter versus summer – the air’s capacity to absorb heat changes subtly.
So, standard air has a specific heat value of roughly 1 kJ/kg·K, but that can change depending on factors like humidity and altitude. To get a better idea of how these factors play out in a specific location, check out this resource on standard air Erie, PA , which will give you a more localized understanding. Ultimately, knowing the specific heat value is crucial for various engineering and meteorological calculations, especially when dealing with regional variations.
Temperature and Pressure Range for Standard Values
Standard specific heat values for air are usually defined within a specific range of temperature and pressure. These values are most accurate under standard atmospheric conditions, typically around 15°C (59°F) and 1 atmosphere of pressure (101.325 kPa). Outside of this range, deviations become more pronounced. For example, at very high altitudes where pressure is significantly lower, or at extremely high or low temperatures, the specific heat capacity will deviate from the standard value.
These deviations are usually accounted for through more complex equations that consider the effects of temperature and pressure. Engineers and scientists working with these extreme conditions need to use these more sophisticated models for accurate calculations.
Applications of Specific Heat Capacity Data
Knowing the specific heat capacity of standard air is crucial for numerous engineering and scientific applications. This seemingly simple value allows for accurate predictions and calculations across various fields, leading to more efficient designs and better understanding of complex systems. The following examples highlight its importance in different contexts.
HVAC System Calculations
Accurate calculation of heating and cooling loads in HVAC (Heating, Ventilation, and Air Conditioning) systems relies heavily on the specific heat capacity of air. Consider a scenario designing an HVAC system for a large office building. To determine the amount of energy required to heat or cool a specific volume of air within the building to a desired temperature, engineers utilize the following formula: Q = mcΔT, where Q is the heat energy transferred, m is the mass of air, c is the specific heat capacity of air, and ΔT is the temperature change.
By inputting the known volume of the building’s air space, the density of air (dependent on pressure and temperature), and the desired temperature difference, engineers can calculate the necessary heating or cooling capacity of the HVAC system. Without accurate specific heat capacity data, the system’s design would be inefficient, potentially leading to either inadequate climate control or excessive energy consumption.
For instance, underestimating the specific heat capacity could lead to a system that’s too small to effectively heat or cool the space, while overestimating it could result in an unnecessarily large and expensive system.
Aerospace Industry Applications
In the aerospace industry, understanding the specific heat capacity of air is vital for several reasons. Aircraft design relies on accurate calculations of aerodynamic heating during flight, especially at high speeds. As an aircraft moves through the air, friction generates heat, and the specific heat capacity of the air surrounding the aircraft directly impacts the temperature increase. This information is critical in designing effective thermal protection systems for aircraft and spacecraft.
Furthermore, the specific heat capacity of air is crucial for designing and optimizing the performance of aircraft engines and their cooling systems. The heat generated by combustion in the engine needs to be managed effectively, and accurate knowledge of the air’s heat capacity allows engineers to design efficient cooling systems to prevent engine damage.
Meteorological Applications
Meteorologists use specific heat capacity data to model atmospheric processes and weather patterns. The specific heat capacity of air influences the rate at which the atmosphere heats up or cools down, which is crucial for understanding and predicting temperature changes. For example, large bodies of water have a high specific heat capacity compared to land. This difference influences the temperature variations between land and sea, leading to the formation of sea breezes and land breezes.
Furthermore, specific heat capacity data is used in models that predict the movement of air masses and the development of weather systems. Accurate predictions of temperature changes are essential for forecasting weather events, such as heat waves, cold snaps, and storms. The energy required to change the temperature of a large air mass is directly proportional to its specific heat capacity, making it a critical parameter in weather forecasting models.
Comparing Specific Heat Capacities
Standard air, a mixture primarily of nitrogen and oxygen, has a specific heat capacity that’s influenced by its composition and the conditions under which it’s measured. Comparing its specific heat capacity to other common gases helps us understand the variations in energy storage and transfer properties of different gases. This comparison is crucial in various engineering applications, from designing efficient HVAC systems to optimizing combustion processes.
The specific heat capacity of a gas can be expressed in two ways: at constant pressure (Cp) and at constant volume (Cv). The difference between these two values is related to the gas constant, a fundamental property that reflects the relationship between pressure, volume, and temperature for ideal gases. Let’s explore these values for standard air and other gases, and see how this difference plays out.
Specific Heat Capacity Comparison of Gases
The table below shows the specific heat capacities (at constant pressure, Cp, and at constant volume, Cv) and densities for standard air and several other common gases at standard temperature and pressure (STP, approximately 0°C and 1 atm). Note that these values can vary slightly depending on the source and the exact composition of the gas mixture.
| Gas | Cp (kJ/kg·K) | Cv (kJ/kg·K) | Density (kg/m³) |
|---|---|---|---|
| Standard Air | 1.005 | 0.718 | 1.225 |
| Nitrogen (N₂) | 1.040 | 0.743 | 1.251 |
| Oxygen (O₂) | 0.918 | 0.658 | 1.429 |
| Carbon Dioxide (CO₂) | 0.846 | 0.657 | 1.977 |
Implications of Specific Heat Capacity Differences
The differences in specific heat capacities among these gases stem from their molecular structures and the ways their molecules store energy. For instance, diatomic gases like nitrogen and oxygen have more degrees of freedom for energy storage (rotational and vibrational) compared to monatomic gases. This results in higher specific heat capacities. Carbon dioxide, being a triatomic molecule, has even more complex vibrational modes, leading to further variations in its specific heat capacity.
The higher density of CO2 also contributes to its lower specific heat capacity per unit volume.
These differences have significant implications in various applications. For example, in internal combustion engines, the specific heat capacity of the combustion gases directly impacts the engine’s efficiency and performance. In HVAC systems, the specific heat capacity of air is crucial for determining the heating and cooling loads required to maintain a desired temperature.
Relationship Between Cp, Cv, and the Gas Constant for Standard Air
For an ideal gas, the relationship between Cp, Cv, and the gas constant (R) is given by:
Cp – Cv = R
For standard air, using the values from the table above (Cp ≈ 1.005 kJ/kg·K and Cv ≈ 0.718 kJ/kg·K), we can calculate the gas constant:
R ≈ 1.005 kJ/kg·K – 0.718 kJ/kg·K ≈ 0.287 kJ/kg·K
This value is consistent with the generally accepted value of the gas constant for air. This relationship highlights the fundamental thermodynamic properties of gases and their connection to specific heat capacities.
Illustrative Example: Adiabatic Process

Let’s consider a practical example of an adiabatic process involving standard air – the compression stroke in a diesel engine. This process happens so quickly that there’s negligible heat exchange with the surroundings. We’ll explore the temperature and pressure changes, and how the specific heat capacity of air plays a crucial role in determining the final state.Imagine a piston-cylinder system containing standard air.
The piston is initially at a low position, meaning the air has a relatively large volume and low pressure. The temperature, let’s say, is 300 Kelvin (approximately 27°C or 80°F). Now, the piston is rapidly compressed, decreasing the volume significantly.
Adiabatic Compression of Standard Air
During this rapid compression, the air molecules collide more frequently, increasing their kinetic energy. This increased kinetic energy manifests as a rise in temperature. Because the process is adiabatic, no heat is exchanged with the environment; all the work done on the system goes into increasing the internal energy of the air, leading directly to a temperature increase. Simultaneously, the pressure increases dramatically due to the reduced volume and increased molecular collisions.
Imagine the air molecules, initially relatively spread out and moving with moderate speeds, being forced closer together, their speeds significantly increasing. This forceful compression is the primary driver of the temperature and pressure increase. The lack of heat transfer is key; the energy is transformed solely from mechanical work (compression) to increased internal energy (higher temperature).
Calculating Final Temperature and Pressure
The specific heat capacity of standard air (at constant volume, Cv, or constant pressure, Cp, depending on the specifics of the compression) is crucial for calculating the final temperature and pressure after adiabatic compression. We use the adiabatic process equation:
P1V 1γ = P 2V 2γ
where P 1 and V 1 are the initial pressure and volume, P 2 and V 2 are the final pressure and volume, and γ (gamma) is the ratio of specific heats (Cp/Cv) for air, approximately 1.
4. We can also relate temperature and volume using another adiabatic equation
T1V 1γ-1 = T 2V 2γ-1
where T 1 and T 2 are the initial and final temperatures. Knowing the initial conditions (T 1, P 1, V 1), the compression ratio (V 1/V 2), and the specific heat ratio (γ), we can solve for the final temperature (T 2) and pressure (P 2). For example, if the compression ratio is 15:1, a significant temperature increase (potentially exceeding 1000 Kelvin) and a substantial pressure increase would be observed.
This illustrates the power of adiabatic compression and highlights the importance of knowing the specific heat capacity of air for accurate prediction of these changes. The same principles apply in reverse for adiabatic expansion, with a decrease in temperature and pressure.
So, there you have it – a deep dive into the often-overlooked world of standard air’s specific heat. From defining what exactly constitutes “standard air” to exploring its applications in various fields, we’ve covered the essentials. Understanding these values isn’t just about theoretical physics; it’s about practical applications in real-world systems. Remember, the next time you’re cranking up the AC or marveling at a jet plane, you’ll have a deeper appreciation for the subtle yet powerful role of specific heat in making it all work.
Top FAQs: Standard Air Has A Specific Heat Value Of
What are the typical units for specific heat capacity?
Common units include Joules per kilogram-Kelvin (J/kg·K) and kilojoules per kilogram-degree Celsius (kJ/kg·°C).
Why is there a difference between Cp and Cv?
Cp (constant pressure) is higher than Cv (constant volume) because at constant pressure, some energy goes into doing work (expansion) as well as increasing internal energy (temperature).
How does humidity affect specific heat?
Higher humidity increases the specific heat capacity of air because water vapor has a higher specific heat than dry air.
Where can I find more precise specific heat values for air?
Thermodynamic property tables and engineering handbooks provide more detailed and temperature-dependent data.
